In 2024, the Metal Functional Materials Research Group led by Professor Zhu Shengli from the School of Materials Science and Engineering at Tianjin University, in collaboration with Professor Wu Shuilin from Peking University, published a research paper titled "Rapid and Effective Treatment of Chronic Osteomyelitis by Conductive Network-Like MoS2/CNTs Through Multiple Reflection and Scattering Enhanced Synergistic Therapy" in the internationally renowned journal Bioactive Materials (IF: 18.0). Jin Liguo, a doctoral student from the class of 2021, is the first author of this paper.
Chronic osteomyelitis (COM) is a chronic, persistent disease of the bone marrow, periosteum, bone cortex, and surrounding tissues caused by pathogenic bacteria or aseptic inflammation from host tissue necrosis, acute osteomyelitis following trauma, diabetic foot, implant surgery infection, or blood dissemination, and associated with bone destruction and dead bone formation. It is difficult to treat, but if not treated appropriately and in a timely manner, it can lead to life-threatening conditions, such as joint dysfunction and physical disability. The traditional clinical treatments for COM include surgical debridement and systemic antibiotic injection, but these have high recurrence rates. Debridement has always been a treatment for osteomyelitis. It involves the removal of all dead bones and any infected bone or soft tissue to prevent unnecessary vascularization and loss of stability. Clinical treatment requires the long-term use of one or several antibiotics to prevent the continued deterioration of COM, which often has various side effects, including drug resistance and nephrotoxicity. It is not difficult to see that for the multiple difficulties of treating COM, antibiotic treatment alone is difficult to completely clear, and the challenge of resistance ensues with the long-term use of antibiotics. To address the antibiotic resistance crisis, antibiotic-free treatment strategies are being developed.
Microwave (MV) therapy is a promising therapeutic tool because it can penetrate biological tissue with negligible attenuation of the surrounding healthy cells and tissues. Carbon nanotubes (CNTs) are characterized by low density, excellent electrical conductivity, and high specific surface area. However, alone CNTs exhibit poor MW absorption properties due to their high conductivity, leading to impedance mismatch. The combination of CNTs with different dielectric properties of materials typically builds heterogeneous interfaces and produces abundant interfacial dipoles. Due to the large specific surface area and interfacial polarization at defect sites, it has been found that MoS2 has significant dielectric and absorption properties. However, its interfacial impedance matching is limited due to its weak conductivity. It is difficult to obtain excellent electromagnetic properties from MoS2 alone, so it is necessary to add highly conductive materials to it to improve its performance. Thus, we designed a combination of CNTs and a flake nanoflower of MoS2, which could produce the synergistic effects of impedance matching, conduction loss, polarization loss, and multiple scattering. Under MW irradiation, MoS2/CNTs produced large amounts of heat and reactive oxygen species (ROS) for the rapid treatment of Staphylococcus aureus (S. aureus)-infected COM through a combination of MW thermos therapy (MTT) and MW dynamics therapy (MDT).

Figure 1. The combination of MTT and MDT for the treatment of COM with deep tissue infection was achieved using MoS2 and CNTs to form heterojunction MoS2/CNTs, which generated heat and ROS under MW irradiation.
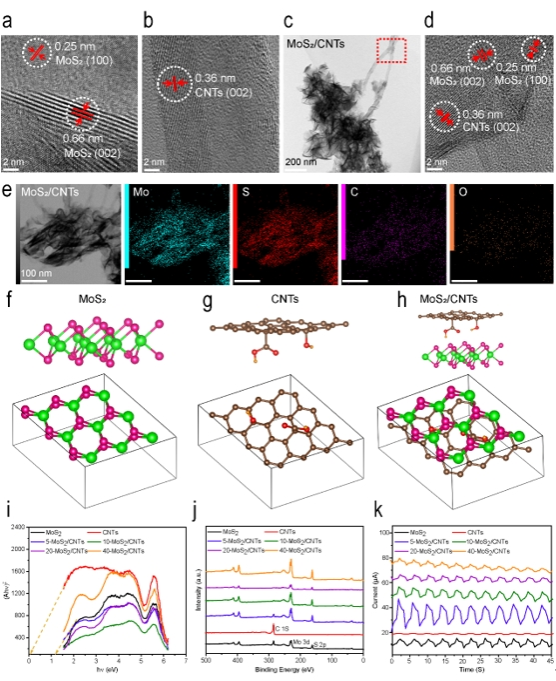
Figure 2. (a) HRTEM image of MoS2 (Scale bar: 2 nm). (b) HRTEM image of CNTs (Scale bar: 2 nm). (c) TEM image of MoS2/CNTs (Scale bar: 200 nm). (d) HRTEM image of MoS2/CNTs (Scale bar: 2 nm). (e) TEM mapping images of MoS2/CNTs (Scale bar: 100 nm). (f) The atomic structure of MoS2. The atoms of Mo and S were represented in green and pink, respectively. (g) The atomic structure of CNTs. The atoms of C, H, and O were represented in brown, orange, and red, respectively. (h) The atomic structure of MoS2/CNTs. The atoms of Mo, S, C, H, and O were represented in green, pink, brown, orange, and red, respectively. (i) UV-Vis spectra of MoS2, CNTs and MoS2/CNTs. (j) XPS spectra of MoS2, CNTs and MoS2/CNTs. (k) MW current of MoS2, CNTs and MoS2/CNTs.

Figure 3. (a) RL spectra of MoS2. (b) RL spectra of 5-MoS2/CNTs. (c) RL spectra of 10-MoS2/CNTs. (d) RL spectra of 20-MoS2/CNTs. (e) RL spectra of 40-MoS2/CNTs. (f) 3D RL spectra of MoS2. (g) 3D RL spectra of 5-MoS2/CNTs. (h) 3D RL spectra of 10-MoS2/CNTs. (i) 3D RL spectra of 20-MoS2/CNTs. (j) 3D RL spectra of 40-MoS2/CNTs. (k) The contour RL spectra of MoS2. (l) The contour RL spectra of 5-MoS2/CNTs. (m) The contour RL spectra of 10-MoS2/CNTs. (n) The contour RL spectra of 20-MoS2/CNTs. (o) The contour RL spectra of 40-MoS2/CNTs.
In this paper, we report on an MW-assisted sterilization strategy. COM caused by S. aureus infection was treated using MW-responsive MoS2/CNTs. MoS2/CNTs had excellent MW absorption properties, mainly due to the promotion of impedance matching and enhanced interfacial polarization. In vitro and in vivo experiments demonstrated that MoS2/CNTs had excellent cytotoxicity, biosafety and antibacterial activity. It was also shown that under MW irradiation, MoS2/CNTs could generate a mass of heat and ROS. The antibacterial rate of 20-MoS2/CNTs against S. aureus and E. coli reached 99.97% under MW irradiation. The mechanism was explained by MW network vector analysis, density of states (DOS), oxygen adsorption energy, differential charge, and finite element (FEM) of heat and ROS generation under MW irradiation. MoS2/CNTs could store a large amount of charge and release more electrons easily. Under MW irradiation, ROS was produced by the combination of oxygen adsorbed by a large number of high-energy electrons. Moreover, the heterogeneous interface of MoS2/CNTs produced abundant interfacial dipoles, which significantly attenuated MW energy to generate heat. After 56 days of COM treatment with 20-MoS2/CNTs, excellent antibacterial, anti-inflammatory, and osteogenic abilities were demonstrated in vivo by blood routine, blood biochemical tests, hematoxylin-eosin (H&E) staining, Masson staining, alizarin red staining, immunohistochemical staining, and X-ray scanning tests. The combined MTT-MDT developed is an important step forward for MW therapy in this study.
文章链接:https://doi.org/10.1016/j.bioactmat.2023.08.005
文章DOI号:10.1016/j.bioactmat.2023.08.005