Recently, Professor Zhu Shengli's research team on metal functional materials from the School of Materials Science and Engineering, Tianjin University, published a research paper titled "Dynamically Restructuring Nanoporous Cu-Co Electrocatalyst for Efficient Nitrate Electroreduction to Ammonia" in the internationally renowned materials journal ACS Catalysis. PhD student Zhou Xue, from the 2020 cohort, is the first author of the paper.
Electrocatalytic nitrate reduction to ammonia (NITRR) is considered an effective method for treating nitrate wastewater and green ammonia (NH3) synthesis. However, the multi-electron/proton transfer processes in this reaction result in slow reaction kinetics, especially in the hydrogenation step, limiting the NH3 yield and Faradaic efficiency. To address this issue, this study reports a nanoporous Cu-Co electrocatalyst for efficient NITRR. The catalyst achieves high NH3 yield of 689.8 mmol h−1 gcat−1 and 2092.0 mmol h−1 gcat−1, with Faradaic efficiencies of 85.3% and 91.5% in electrolytes containing 200 and 1400 ppm NO3−-N, respectively. In situ Raman spectroscopy reveals that, during the NITRR process, the CuCo alloy undergoes in situ reconstruction through a chemical/electrochemical reaction equilibrium with NO3−, forming a Cu/CoOOH heterojunction catalyst. This Cu/CoOOH heterojunction catalyst exhibits stronger binding to the *NO2 intermediate, reducing the energy barrier for the rate-determining step of *NO2H formation and effectively promoting the reduction of NO3− to NH3. This study elucidates the dynamic structural evolution mechanism of the cathode catalyst during the electrochemical reaction process, providing insights for designing efficient electrocatalysts for nitrate reduction reactions.
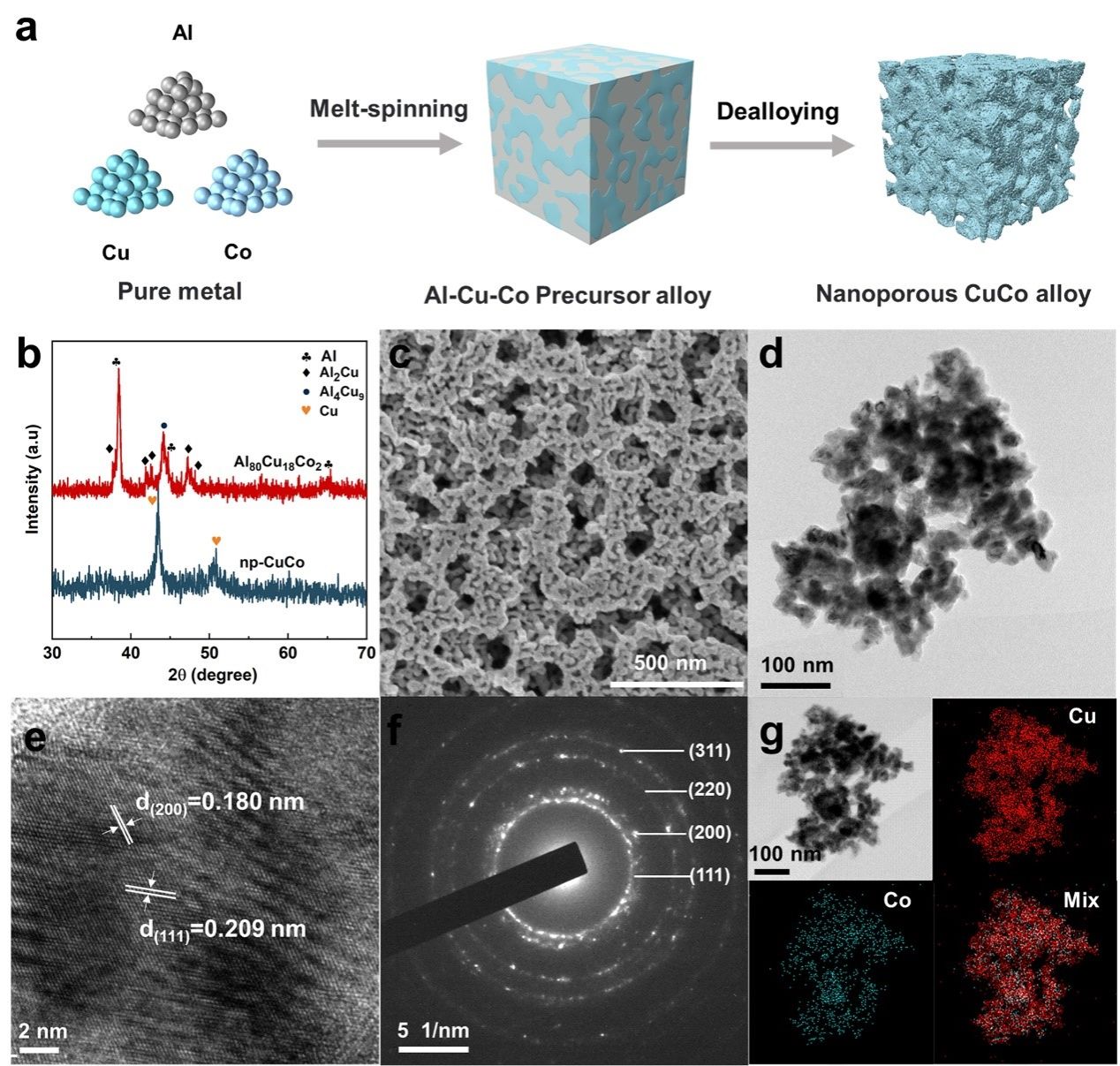
Figure 1. Schematic diagram of the preparation process and structural characterization of the nanoporous CuCo catalyst.
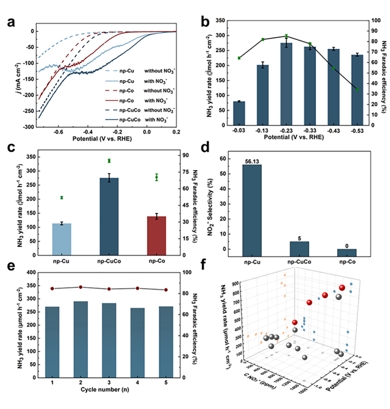
Figure 2. NITRR performance of the nanoporous CuCo catalyst.
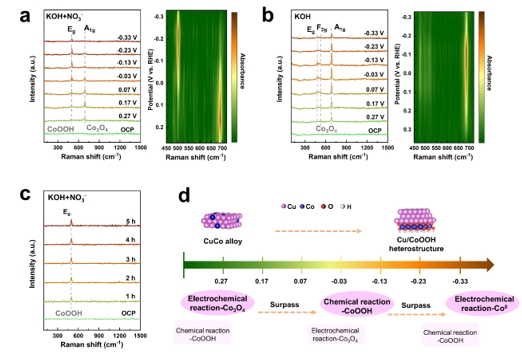
Figure 3. In situ Raman spectra of the np-CuCo catalyst at different applied potentials in 1 M KOH (a)with and (b)without NO3− electrolyte; (c) In situ Raman spectra of the np-CuCo catalyst during immersion in the electrolyte; (d) Schematic illustration of the structural evolution mechanism of np-CuCo during the NITRR process.
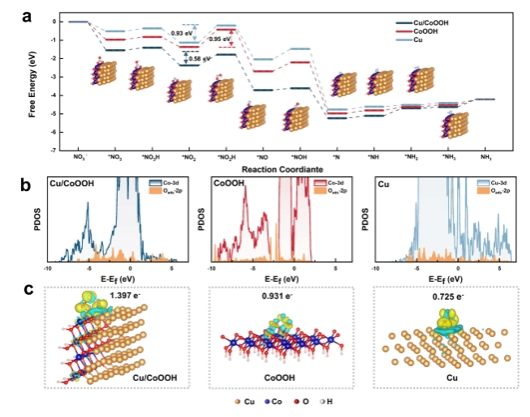
Figure 4. Theoretical simulation and calculation of the Cu/CoOOH heterojunction catalyst in the NITRR reaction.
文章链接:https://pubs.acs.org/doi/10.1021/acscatal.4c03336
文章DOI号:10.1021/acscatal.4c03336