自支撑纳米多孔Co-Cr-Fe-Ni-Nb高熵氧化物析氧电催化剂
图文摘要
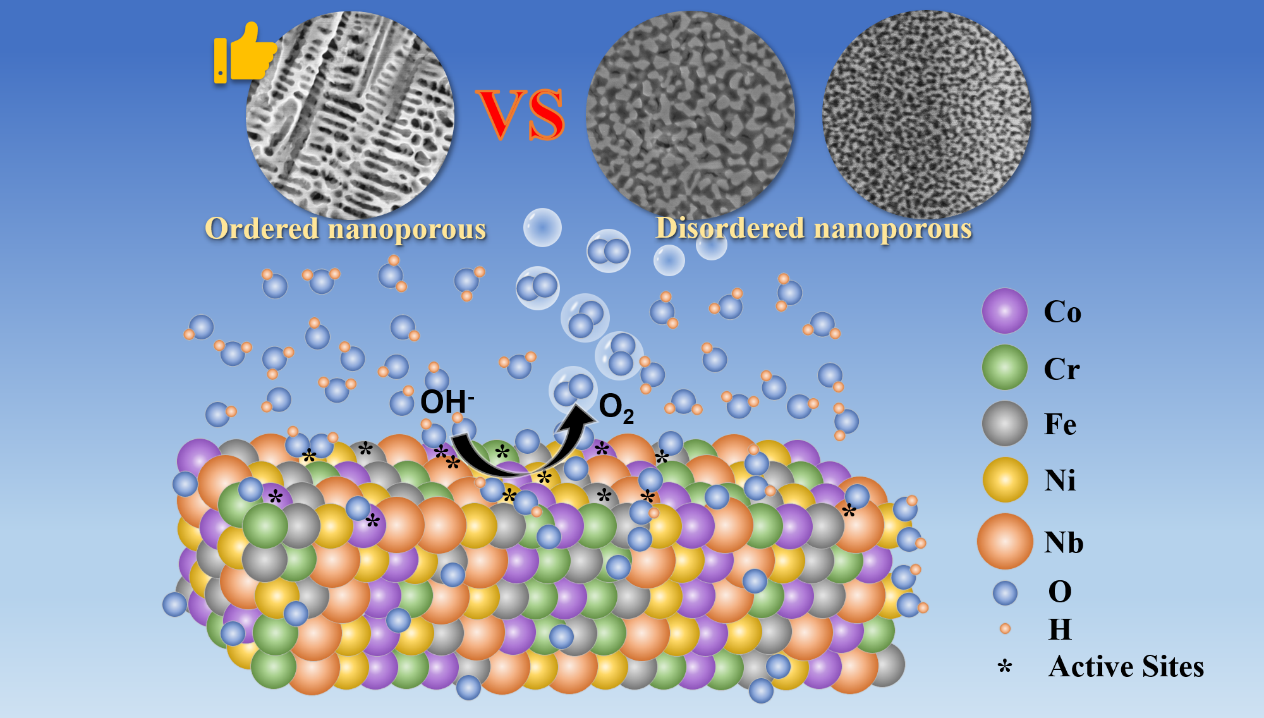
In this study, a self-supporting nanoporous Co-Cr-Fe-Ni-Nb high-entropy alloy electrocatalyst for water electrolysis was prepared using the melt-spinning and chemical dealloying methods. Thanks to the ultra-fast reaction kinetics enabled by the ordered nanoporous structure and the diverse active intermediates provided by the high-entropy alloy, this electrocatalyst exhibits excellent oxygen evolution reaction (OER) catalytic performance in 1 M KOH. This work offers valuable insights into the design and fabrication of nanoporous high-entropy alloy electrocatalysts.
研究背景
In the 21st century, the continuous growth of global energy demand and the extensive use of fossil fuels have led to environmental pollution and resource depletion. Hydrogen energy, as a clean and sustainable energy source, has garnered significant attention. Among hydrogen production methods, water electrolysis offers advantages such as abundant raw materials and low costs. However, the reactions involved in water electrolysis face significant energy barriers, necessitating the development of efficient and stable catalysts.Water electrolysis comprises two half-reactions: the oxygen evolution reaction (OER) and the hydrogen evolution reaction (HER). OER, involving a complex four-electron transfer process, is particularly challenging as the activity and stability of its intermediates govern the catalytic reaction kinetics. Precious metal catalysts like RuO2 and IrO2 exhibit high catalytic activity but are limited by their scarcity and high costs, restricting their large-scale application.In contrast, transition metals such as Fe, Co, and Ni have shown great promise in electrocatalysis due to their low cost, abundance, and high catalytic activity. Dealloying techniques can be used to create nanoporous structures, which enhance specific surface area and conductivity, further improving catalytic performance. However, the mechanical properties of nanomaterials are often inadequate, and formulating them into electrode slurries for coating on supports can mask active sites and reduce long-term durability.To address these challenges, self-supporting electrode materials are being developed to improve stability. High-entropy alloys (HEAs) exhibit exceptional mechanical properties, a unique "cocktail effect," and a flexible compositional design platform. By combining the advantages of nanoporous structures and HEAs, nanoporous high-entropy alloys (np-HEAs) hold great promise as catalysts with both superior mechanical and electrocatalytic performance, making them a compelling candidate for future energy applications.
研究内容
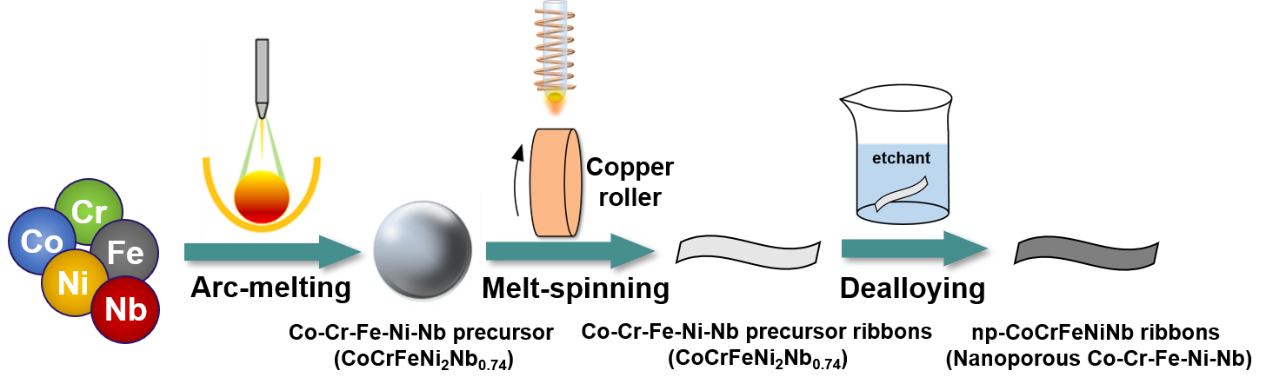
Fig. 1. Schematic illustration of the fabrication process of self-supporting nanoporous Co-Cr-Fe-Ni-Nb (np-CoCrFeNiNb) catalyst.
We prepared a self-supporting nanoporous CoCrFeNiNb high-entropy alloy catalyst (np-CoCrFeNiNb) using melt-spinning and chemical dealloying methods. The study focused on comparing the mechanical properties and OER catalytic performance of catalysts with different morphologies (ordered structures and disordered structures). Additionally, we investigated the reasons behind the superior OER catalytic performance of the ordered nanoporous CoCrFeNiNb high-entropy alloy catalyst in an alkaline environment.
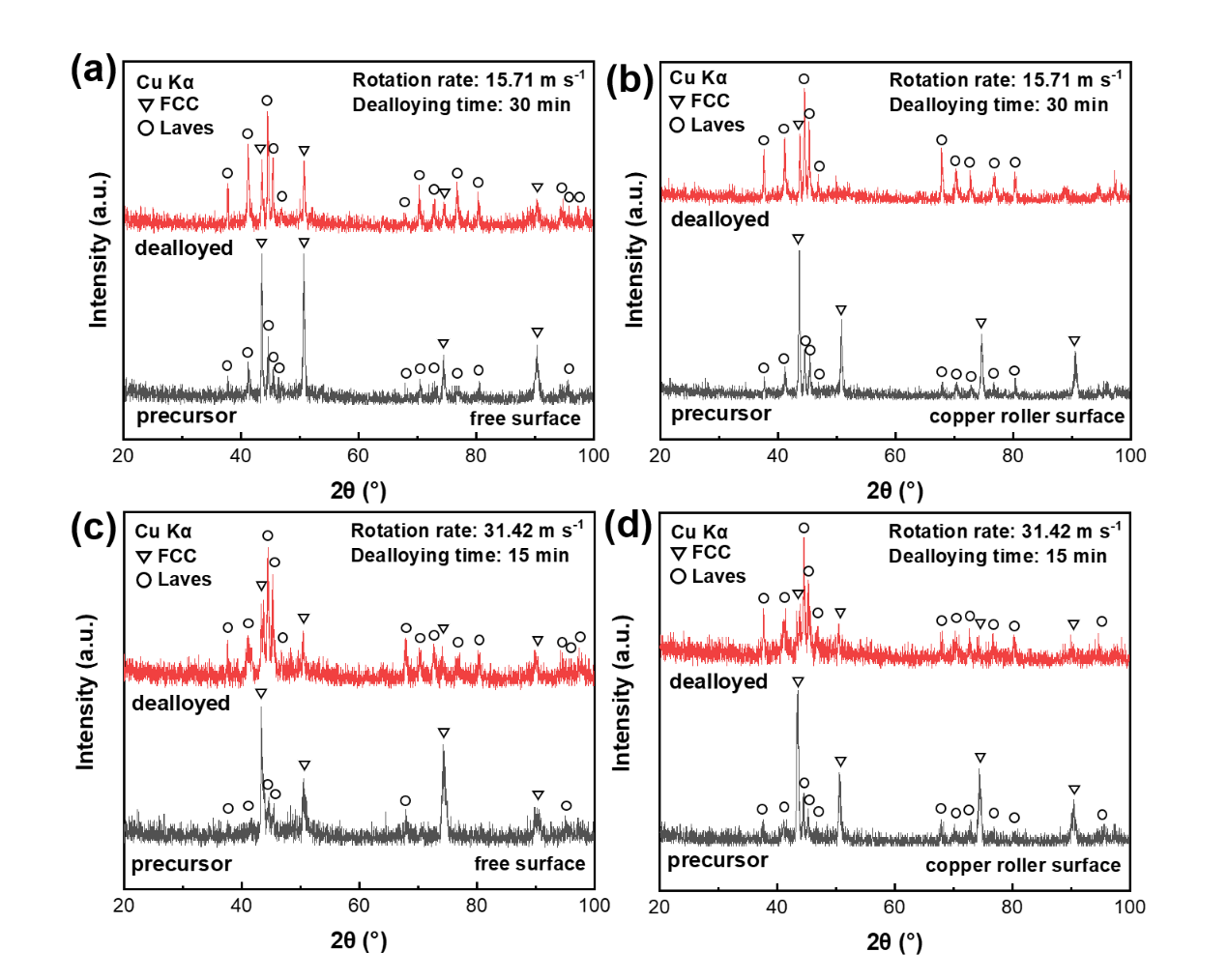
Fig. 2. XRD patterns of (a) the free surface and (b) the copper roller surface of the Precursor-1 sample before and after dealloying, (c) the free surface and (d) the copper roller surface of the Precursor-2 sample before and after dealloying.
The XRD patterns of the free surface and copper roller surface of the nanoporous CoCrFeNiNb high-entropy alloy catalyst before and after dealloying reveal that the catalyst is composed of FCC and Laves phases. During the dealloying process, the FCC phase, which exhibits lower corrosion resistance, is selectively etched, while the Laves phase, with higher corrosion resistance, remains intact.
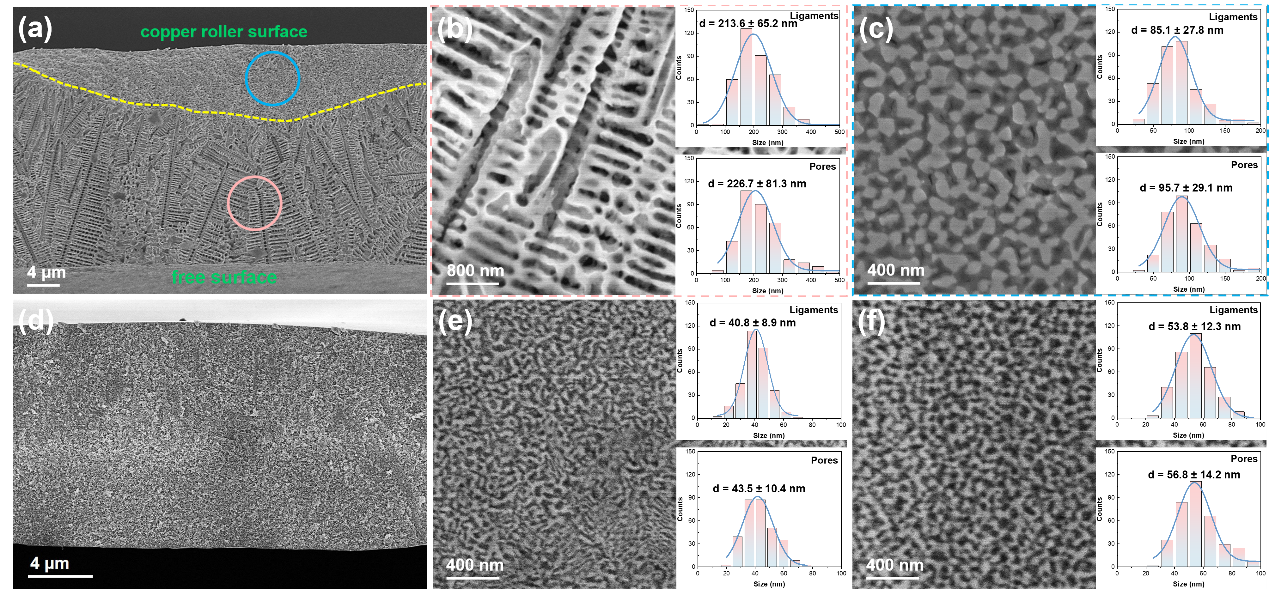
Fig. 3. SEM images of the Precursor-1 sample after dealloying for 30 min of (a) the whole cross-section, (b) the free surface, (c) the copper roller surface, and the Precursor-2 sample after dealloying for 15 min of (d) the whole cross-section, (e) the free surface, and (f) the copper roller surface.
The SEM images of the nanoporous CoCrFeNiNb high-entropy alloy catalyst before and after dealloying illustrate distinct morphological differences between the free surface and the copper roller surface. On the free surface, which cools at a slower rate, atoms undergo migration and aggregation, forming a eutectic microstructure. After dealloying, this results in an ordered nanoporous structure. In contrast, the copper roller surface cools more rapidly, preventing atomic migration and aggregation. The crystals are "frozen" before they can grow, forming a nanocrystalline phase. After dealloying, this leads to the formation of a disordered nanoporous structure.
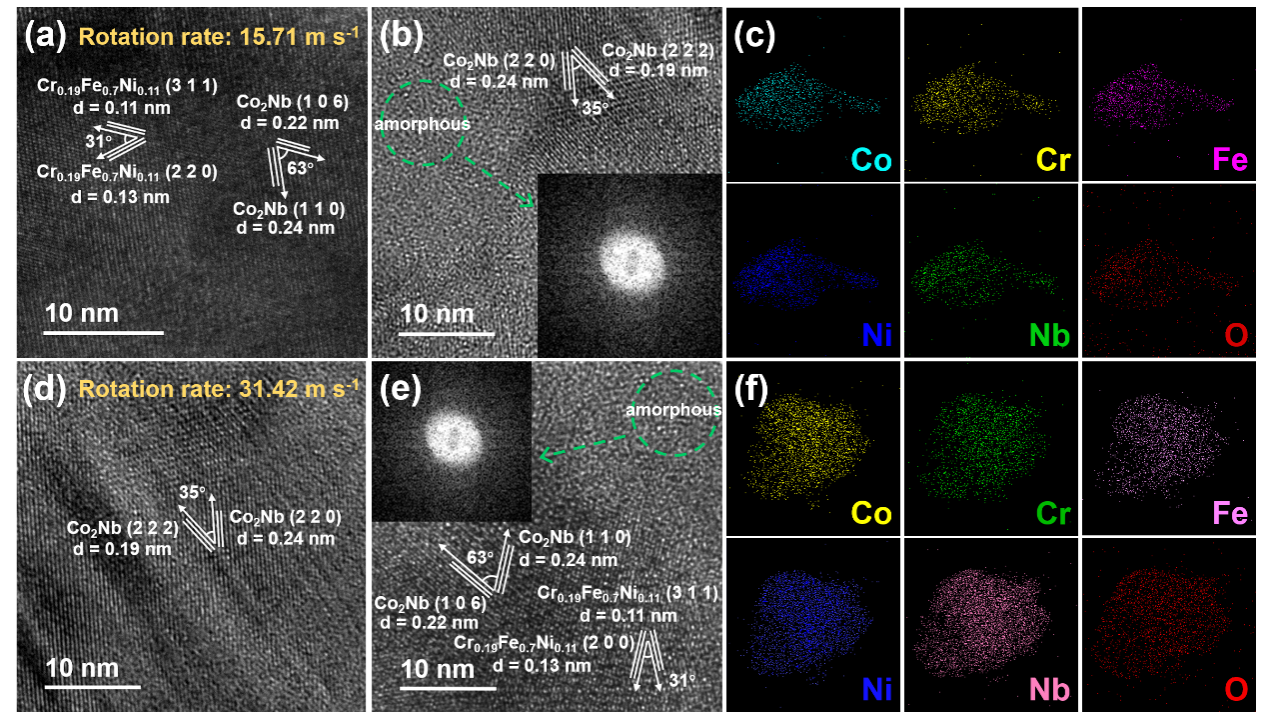
Fig. 4. (a)(b) HRTEM images and (c) elemental mapping of Co, Cr, Fe, Ni, Nb and O of the Precursor-1 sample after dealloying, and (d)(e) HRTEM images and (f) elemental mapping of Co, Cr, Fe, Ni, Nb and O of the Precursor-2 sample after dealloying.
The TEM images of the nanoporous CoCrFeNiNb high-entropy alloy catalyst reveal that the catalyst consists of an FCC phase (Cr₀.₁₉Fe₀.₇₀Ni₀.₁₁) and a Laves phase (Co₂Nb). The presence of amorphous regions is attributed to the significantly larger atomic radius of Nb compared to other elements, causing severe lattice distortions in localized areas of the sample and leading to the formation of amorphous structures. Elemental mapping indicates the uniform distribution of Co, Cr, Fe, Ni, Nb, and O throughout the catalyst, confirming that the sample is a high-entropy oxide.

Fig. 5. High-resolution XPS spectra of (a) Co 2p, (b) Cr 2p, (c) Fe 2p, (d) Ni 2p, (e) Nb 3d, and (f) O 1s in the np-CoCrFeNiNb-1 sample, np-CoCrFeNiNb-2 sample and np-CoCrFeNiNb-4 sample, respectively.
The high-resolution XPS spectra of the nanoporous CoCrFeNiNb high-entropy alloy catalyst show that after dealloying, some metal elements on the catalyst surface are oxidized to higher oxidation states, while others remain in the zero-valence state. This indicates a diverse range of elemental valence states on the catalyst surface.
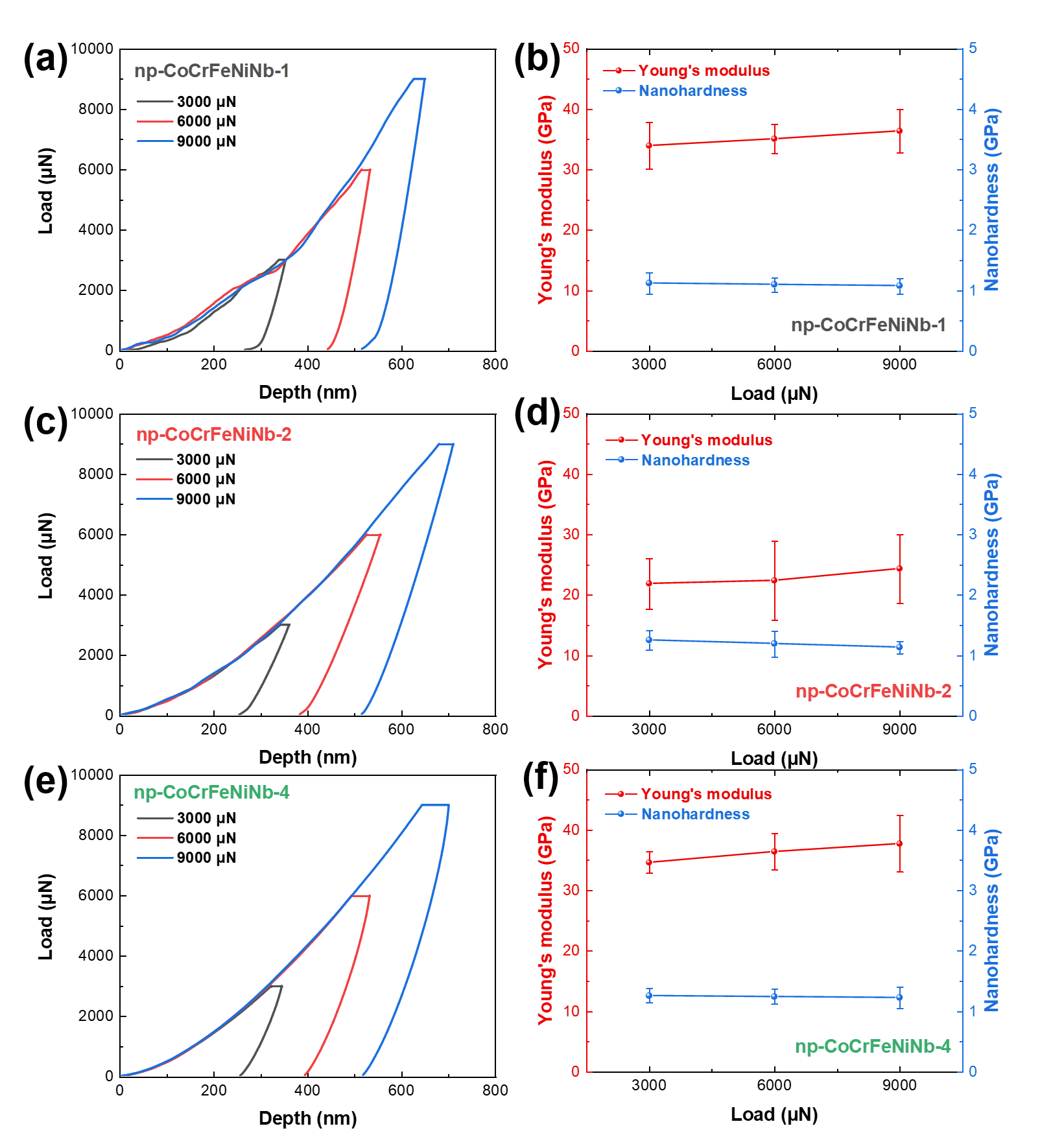
Fig. 6. (a) The indentation load-depth curves of the np-CoCrFeNiNb-1 sample, (b) the nanomechanical properties for the np-CoCrFeNiNb-1 sample, (c) the indentation load-curves of the np-CoCrFeNiNb-2 sample, (d) the nanomechanical properties for the np-CoCrFeNiNb-2 sample, (e) the indentation load-curves of the np-CoCrFeNiNb-4 sample, and (f) the nanomechanical properties for the np-CoCrFeNiNb-4 sample.
The nano-mechanical performance tests of the nanoporous CoCrFeNiNb high-entropy alloy catalyst indicate that its yield strength, calculated using the formula σy=Hardness/2.65\sigma_y = \text{Hardness} / 2.65σy=Hardness/2.65, exceeds 300 MPa. This meets the basic mechanical requirements for self-supporting electrode materials. The high Nb content in the catalyst contributes to its hardness, while the high continuity of the porous structure enhances its modulus.
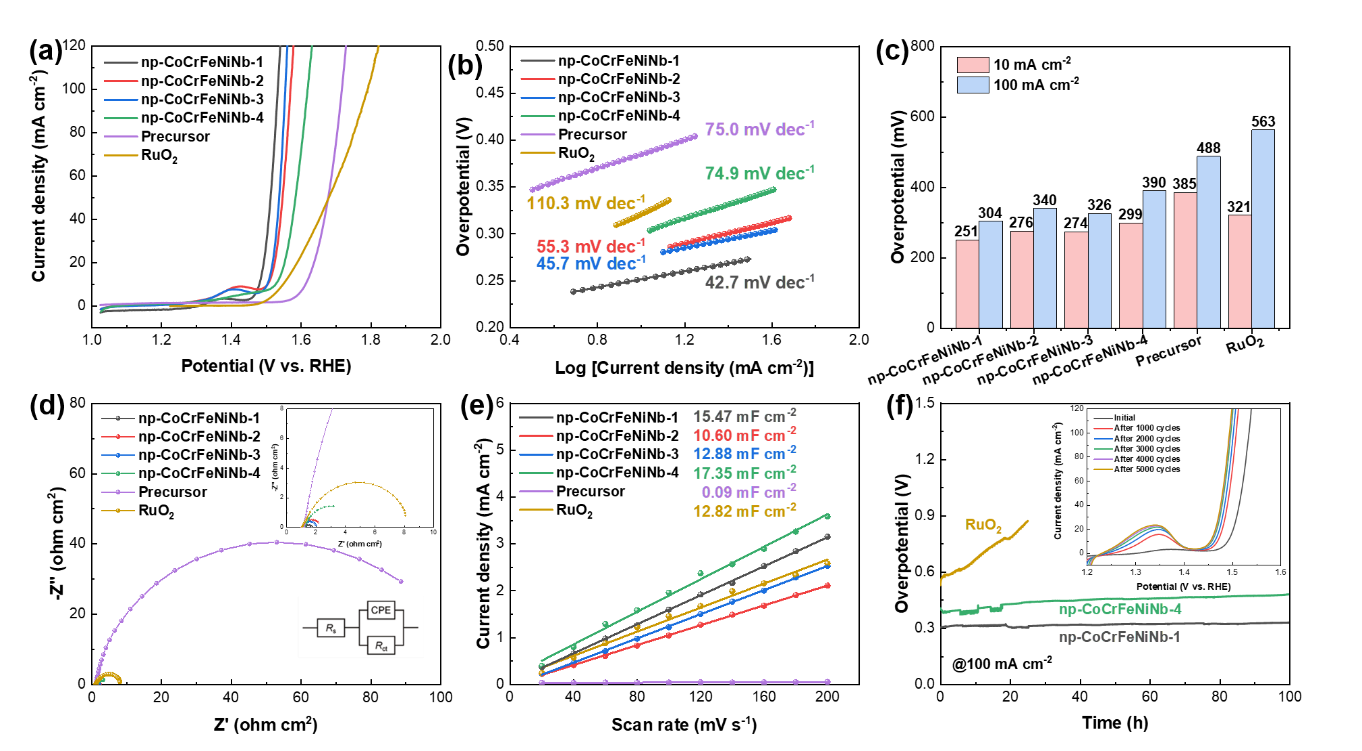
Fig. 7. OER characteristics of prepared catalysts in 1 M KOH. (a) LSV curves of OER. (b) Corresponding Tafel plots are derived from the polarization curves. (c) The overpotential at 10 mA cm–2 and 100 mA cm–2 of catalysts. (d) Nyquist plot. (e) Capacitive current measured at 1.125 V (vs. RHE) plotted against the scan rate. (f) Time-dependent potential profile of the np-CoCrFeNiNb-1 sample, the np-CoCrFeNiNb-4 sample and the RuO2 sample at the constant electrocatalytic current density of 100 mA cm–2 for 100 h.
The electrocatalytic OER performance of the nanoporous CoCrFeNiNb high-entropy alloy catalyst was evaluated in 1 M KOH. The np-CoCrFeNiNb-1 catalyst with an ordered nanoporous structure exhibited overpotentials of 251 mV and 304 mV at current densities of 10 mA cm⁻² and 100 mA cm⁻², respectively, along with a Tafel slope of 42.7 mV dec⁻¹. These metrics are significantly superior to those of the commercial RuO₂ catalyst and clearly outperform the disordered nanoporous np-CoCrFeNiNb-2 and np-CoCrFeNiNb-4 catalysts.
The results demonstrate that the unique ordered nanoporous structure of np-CoCrFeNiNb-1 improves charge transfer kinetics and reaction kinetics. Furthermore, the catalyst exhibits excellent long-term stability, maintaining continuous operation at a current density of 100 mA cm⁻² for over 100 hours.
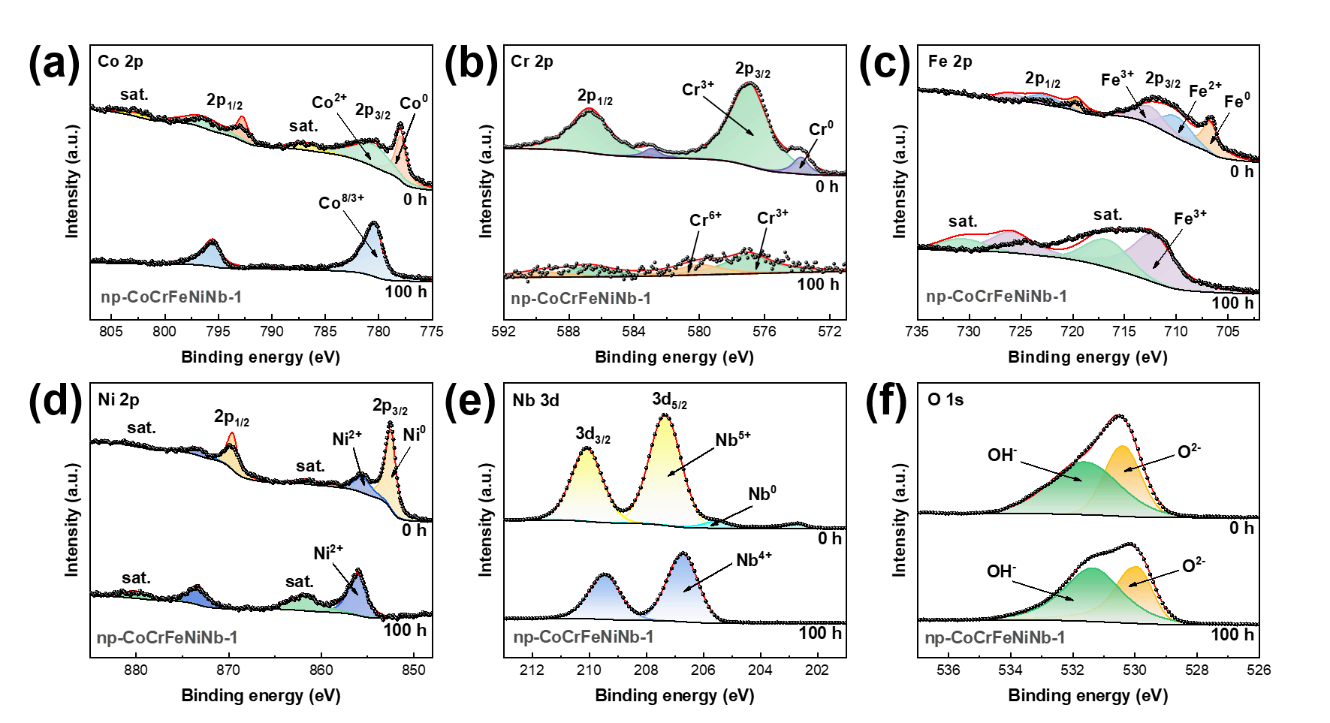
Fig. 8. High-resolution XPS spectra of (a) Co 2p, (b) Cr 2p, (c) Fe 2p, (d) Ni 2p, (e) Nb 3d, and (f) O 1s in the np-CoCrFeNiNb-1 sample at t = 0 h and t = 100 h, respectively.
The oxygen evolution reaction (OER) mechanism of the nanoporous CoCrFeNiNb high-entropy alloy catalyst was investigated. High-resolution XPS spectra before and after stability testing revealed that during the OER process, highly catalytically active species such as Co₃O₄, FeOOH, and Ni(OH)₂ were generated on the catalyst surface, serving as the true active sites for the reaction. Additionally, Cr⁶⁺ and Nb⁴⁺ were formed, which facilitated the charge transfer process and modulated the electronic structure of the catalyst surface, thereby enhancing the charge transfer kinetics and reaction dynamics. The synergistic interaction among the multiple elements collectively improved the OER catalytic activity of the catalyst.
文章信息
第一作者:林宇涵
指导教师:朱胜利
单位:天津大学
本工作发表于:Chemical Engineering Journal
文章题目:Self-supporting high-entropy Co-Cr-Fe-Ni-Nb oxide electrocatalyst with nanoporous structure for oxygen evolution reaction
文章DOI号:10.1016/j.cej.2024.151233