
通过亚稳相工程提高纳米多孔Fe-Pd合金电催化剂的析氢性能
图文摘要
This study utilized an electrochemical dealloying method to fabricate a nanoporous Fe-Pd alloy with a face-centered cubic (fcc) phase structure as an electrocatalyst for the hydrogen evolution reaction (HER). In a 1 M KOH solution, the fcc Fe-Pd catalyst exhibited an overpotential of only 58 mV at 10 mA cm⁻², outperforming the body-centered cubic (bcc) Fe-Pd nanoporous alloy and commercial catalysts. Density functional theory (DFT) calculations revealed that the fcc structure alters the coordination environment and electronic structure of Pd active centers in the Fe-Pd alloy. This shifts the d-band center of Pd atoms away from the Fermi level, weakening the Pd-H interaction and reducing the water dissociation energy barrier. Moreover, the fcc Fe-Pd sample demonstrated excellent mechanical properties, maintaining good catalytic activity even under bending deformation. This work expands the approach to designing HER catalysts by leveraging metastable structures.
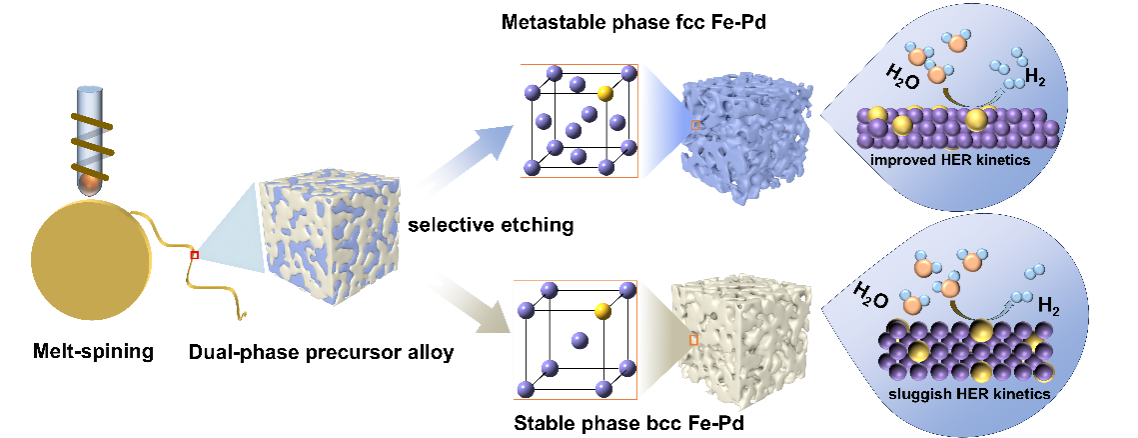
研究背景
Developing cost-effective and high-performance transition metal-based catalysts for water-splitting hydrogen evolution reactions (HER) has been a research focus in the hydrogen energy field. Metastable catalysts, characterized by their unique electronic structures and high-energy non-equilibrium metallic surfaces, exhibit enhanced catalytic activity. However, synthesizing metastable materials is challenging due to their susceptibility to phase transitions from high-energy to low-energy structures.
Alloying is an effective strategy to improve the thermodynamic stability of metastable materials. Leveraging the stabilizing effect of Pd on the metastable phases of iron-based alloys, adding a small amount of Pd to iron-based alloys enables the formation of face-centered cubic (fcc) Fe-Pd alloys at room temperature. Additionally, noble metal Pd, with its high electron affinity and electronegativity, can attract and polarize adjacent transition metal atoms and ligands, adjusting the charge density and distribution, and typically serves as the active center for HER.
Density functional theory (DFT) analysis confirms that metastable engineering enhances the charge density and electronic states of Pd active sites, thereby reducing the energy barriers associated with water adsorption and dissociation.
研究内容
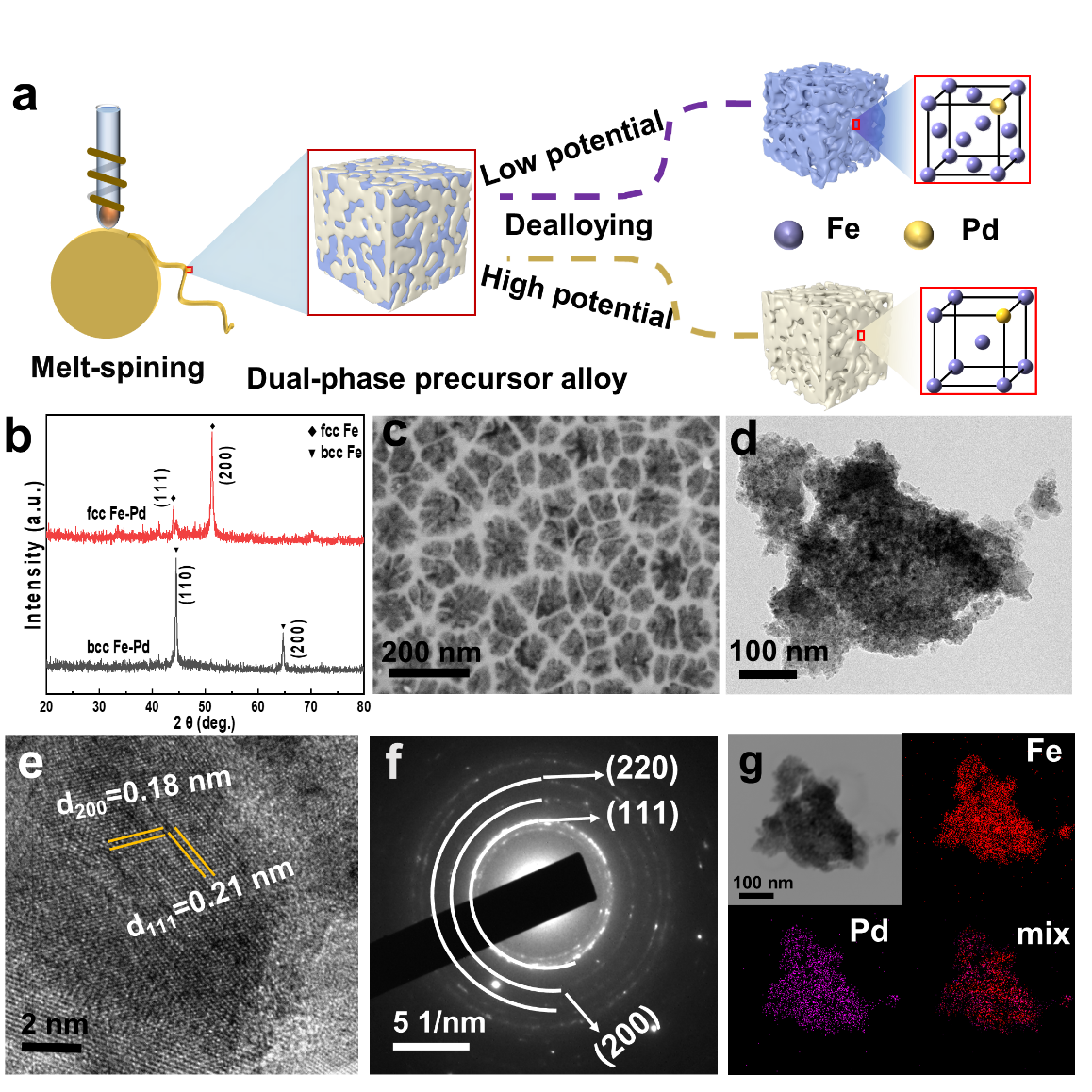
Figure 1. Preparation and characterization of nanoporous Fe-Pd ribbons a). Schematic illustration of Fe based nanopores with adjustable phase formed through electrochemical dealloying strategy. b) XRD patterns of nanoporous Fe-Pd samples. c) Scanning electron microscope images of the surface of fcc Fe-Pd samples. d- e) Bright-field TEM images and high-resolution TEM image. f) The selected area electron diffraction pattern (SAED). g) The corresponding energy dispersive X-ray spectroscopy (EDS) mapping images of fcc Fe-Pd.
By adding a small amount of the noble metal Pd to iron-based alloys, an fcc phase with metastable structures can be obtained at room temperature. Using the electrochemical dealloying method, nanoporous Fe-Pd alloys with different phase compositions were prepared. Due to the differing electrochemical stability of the bcc and fcc phases, phase regulation of the nanoporous layer structure can be achieved by controlling the corrosion potential.
At a dealloying potential of -0.21 V (vs. Ag/AgCl), the more active bcc phase selectively dissolves from the precursor alloy, resulting in a bicontinuous nanoporous fcc Fe-Pd alloy. At a dealloying potential of -0.15 V (vs. Ag/AgCl), both the fcc and bcc phases dissolve simultaneously. However, since the fcc phase is relatively less abundant in the striped regions of the precursor, it fully dissolves after a period of etching, while portions of the bcc phase are retained.
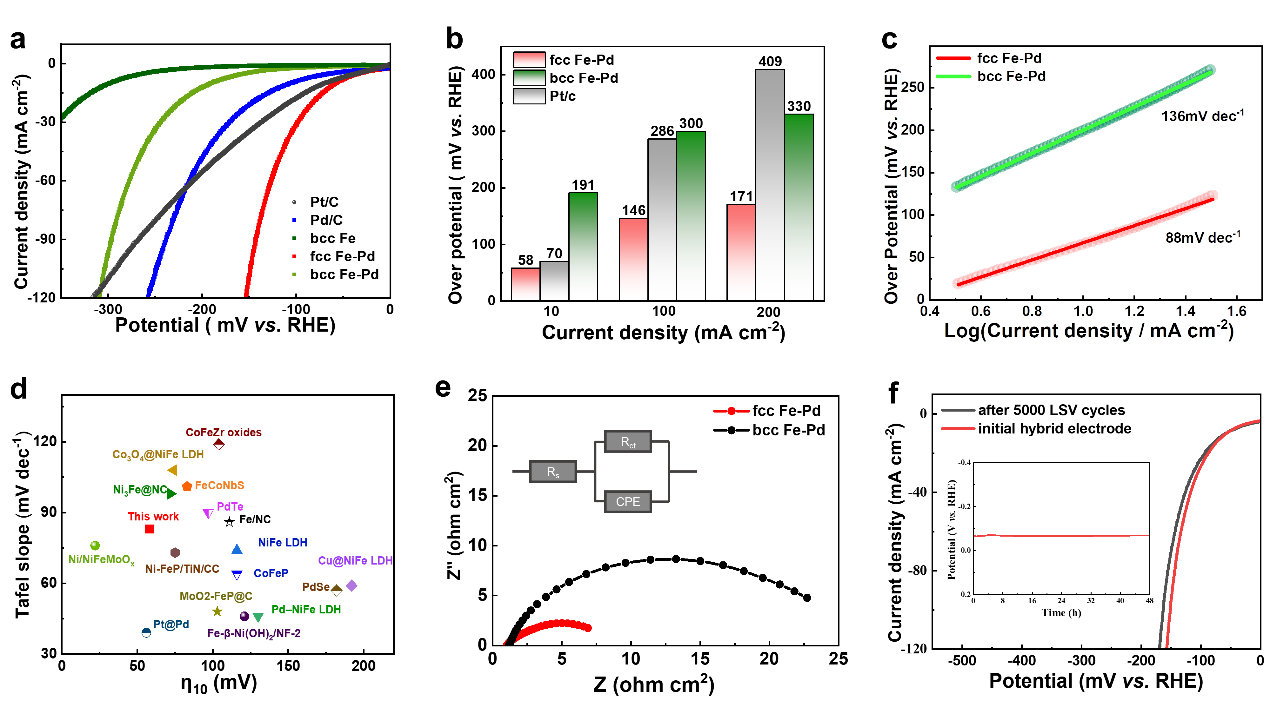
Figure 2. HER electrocatalytic performance of nanoporous Fe-Pd alloys in 1 M KOH solution. a) Linear sweep voltammetry polarization (LSV) curves. b) Overpotential of fcc Fe-Pd at different current densities. c) Tafel plots of fcc Fe-Pd and bcc Fe-Pd. d) Overpotential and Tafel slope comparison with other Fe-base or Pd-base electrocatalysts driven with η10. e) Nyquist plots for different electrodes in 1 M KOH. f) HER polarization curves of fcc Fe-Pd before and after 5000 cycle tests. The illustration shows that the time-dependent HER stability of the fcc Fe-Pd over 48 h at a current density of 10 mA cm-2 in 1.0 m KOH solutions.
In a 1 M KOH solution, the fcc Fe-Pd alloy exhibits an overpotential of only 58 mV at a current density of 10 mA cm⁻², which is significantly lower than that of bcc Fe-Pd (191 mV), commercial Pd/C catalyst (114 mV), and commercial Pt/C catalyst (70 mV). Moreover, the fcc Fe-Pd alloy maintains a low HER overpotential at high current densities, demonstrating its potential for industrial applications.
The Tafel slope values indicate that the Fe-Pd catalysts follow the Volmer step as the rate-determining step for HER. The lower Tafel slope of fcc Fe-Pd suggests improved water dissociation kinetics. Electrochemical impedance spectroscopy (EIS) tests show enhanced electron transfer rates and catalytic kinetics for fcc Fe-Pd. Additionally, the fcc Fe-Pd electrode exhibits excellent catalytic stability even under high current conditions.
These results highlight the outstanding catalytic activity of fcc Fe-Pd for HER under alkaline conditions, emphasizing its potential as an efficient and stable electrocatalyst.
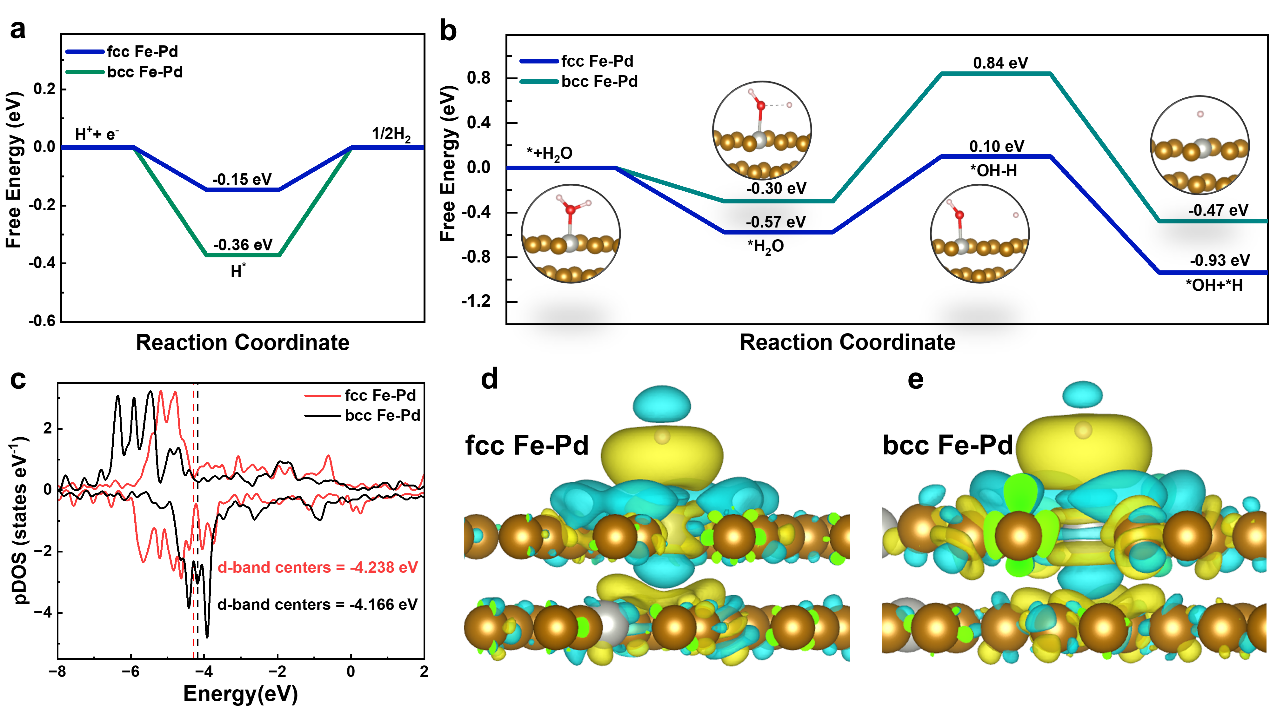
Figure 3. Part of density functional theory (DFT) calculations from first principles. a) hydrogen free energies of systems. b) The H2O dissociation energies. c) pDOS of different samples. d, e) differential charge density of (d) fcc Fe-Pd and (e) bcc Fe-Pd.
The HER mechanism of Fe-Pd alloys was further explored using density functional theory (DFT) calculations, focusing on the Gibbs free energy of hydrogen adsorption (ΔG*_H), which is a key parameter in predicting the theoretical performance of HER catalysts. An ideal HER catalyst should exhibit thermoneutral behavior with a ΔG*_H close to zero, ensuring both efficient H* adsorption and H₂ desorption.
For fcc Fe-Pd, ΔG*_H is calculated to be -0.15 eV, which is much closer to zero compared to -0.36 eV for bcc Fe-Pd. This indicates that the Pd in the fcc phase improves H* adsorption and accelerates H₂ desorption, thereby enhancing HER activity. Moreover, the water dissociation energy barrier for fcc Fe-Pd (0.67 eV) is significantly lower than that of bcc Fe-Pd (1.14 eV), suggesting that the fcc Fe-Pd surface facilitates rapid proton supply.
Projected density of states (pDOS) simulations reveal strong interactions between the Fe 3d and Pd 4d orbitals in the alloy, indicating enhanced electron localization and activity in fcc Fe-Pd. Differential charge density analysis shows that electron density shifts toward Fe atoms in the fcc Fe-Pd catalyst, resulting in fewer electrons available at Pd active sites to interact with H* intermediates. This weakens the interaction between H* and Pd active sites, enabling better H₂O dissociation and H₂ desorption.
Overall, these findings demonstrate that the fcc Fe-Pd alloy improves water splitting and H₂ evolution, thereby promoting superior HER catalytic activity in alkaline media.
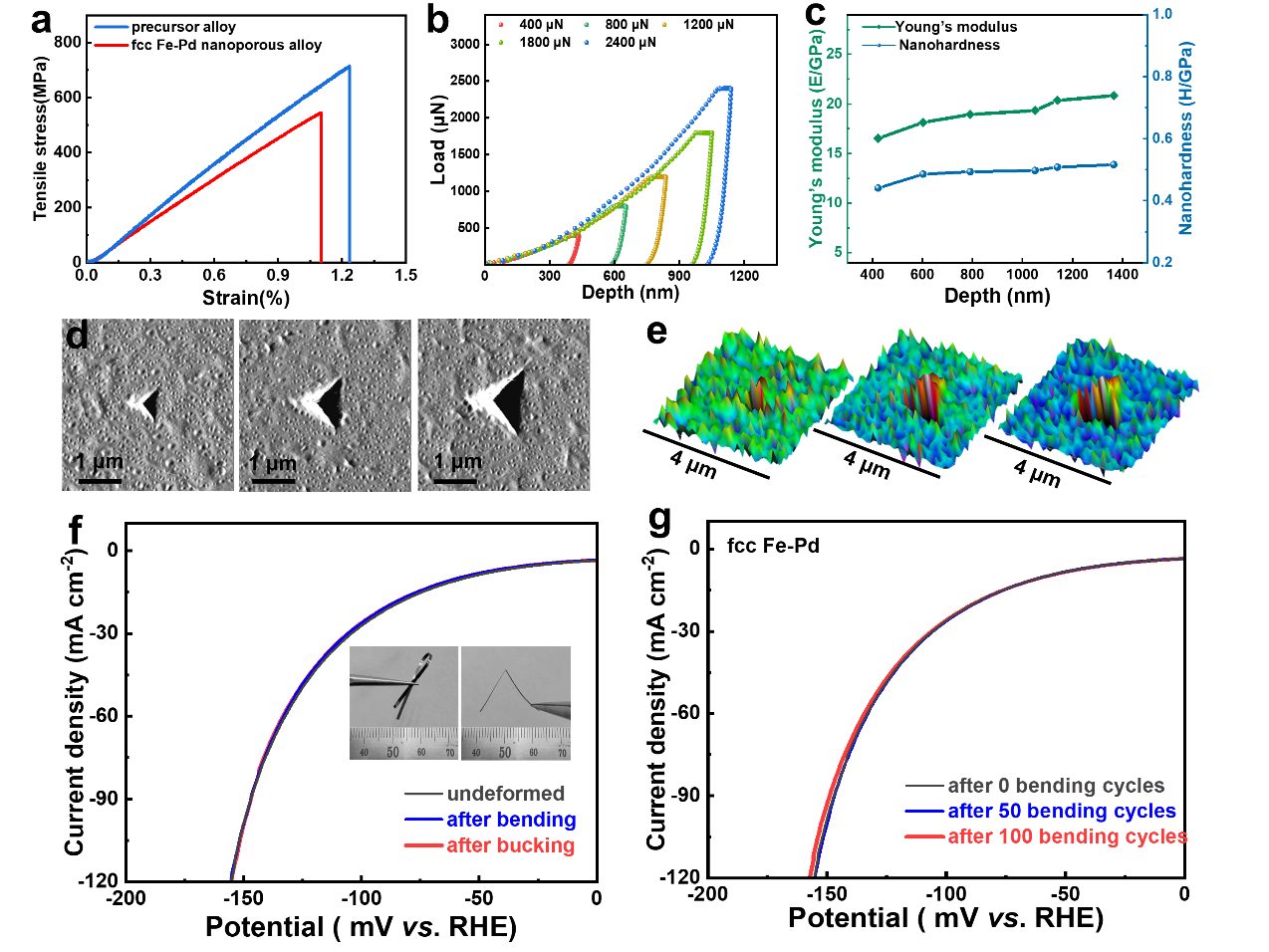
Figure 4. Mechanical measurements of the fcc Fe-Pd ribbon and electrochemical performance under different deformation. a) The tensile stress-strain curves of nanoporous ribbon and precursor ribbon. b) The nanoindentation force-depth curves under different loads. c) Young's Modulus and Nanohardness of fcc Fe-Pd. d, e) The corresponding scanning probe microscope (SPM) images and 3D maps under different loads. f) HER catalytic performance of fcc Fe-Pd under different deformation. g) Comparison of catalytic activity of fcc Fe-Pd electrode after 50 (100) bending cycles with that without bending test.
In practical water splitting processes, flexible electrodes with excellent mechanical properties and robust nanostructures have proven to enhance both electrochemical activity and stability. The tensile stress-strain curve indicates that the ultimate tensile strength of the fcc Fe-Pd strip is 545 MPa, demonstrating that the dealloyed structure retains good mechanical properties. Further nanoindentation tests were conducted to evaluate the mechanical properties of the nanoporous layer. Load-depth curves under varying loads showed that fcc Fe-Pd exhibited excellent mechanical stability under applied loads.
Scanning probe microscopy (SPM) images of the indented region revealed no visible cracks around the indentation, suggesting strong adhesion between the nanoporous surface layer and the underlying matrix. This high interfacial bonding ensures robust mechanical integration and superior charge transfer. Additionally, the catalytic activity of the fcc Fe-Pd electrode remained consistent under various deformation states, indicating that the flexible electrode maintained reliable current output for HER.
Moreover, the fcc Fe-Pd electrode retained its structural integrity and catalytic performance after 100 bending cycles, demonstrating excellent flexibility and recoverability. The effective combination of mechanical robustness and electrocatalytic performance makes fcc Fe-Pd a highly promising candidate material for extensive applications in flexible and durable water-splitting systems.
文章信息
第一作者:李章溢
通讯作者:徐文策*,朱胜利*,崔振铎*
单位:天津大学
本工作发表于:Applied Catalyst B:Environmental
文章题目:Boosting Hydrogen Evolution Performance of Nanoporous Fe-Pd Alloy Electrocatalyst by Metastable Phase Engineering
文章DOI号: 10.1016/j.apcatb.2023.123677