Recently, Professor Zhu Shengli's research team on metal functional materials at the School of Materials Science and Engineering, Tianjin University, published a research paper titled "Towards Scalable Synthesis of Mo2C/MoNi4 Heterojunction Catalysts on Ni Mesh Via Laser Radiation for Efficient H2 Production in Alkaline Electrolysis" in the internationally renowned materials journal Advanced Functional Materials.
Nickel-based catalysts are expected to exhibit better catalytic activity for the Hydrogen Evolution Reaction (HER) in water electrolysis by alloying with other metals/non-metals or by adjusting their chemical structure and morphology. Among them, nickel-molybdenum alloy catalysts are commonly used as HER catalysts, but their catalytic activity still needs further improvement. An effective solution is to introduce a second phase to form heterogeneous structural interfaces based on the original materials. This can provide more possibilities for regulating the adsorption/desorption energy of H*, significantly enhancing the catalytic performance of the materials through synergistic effects. Research results indicate that Mo2C is an excellent HER electrocatalyst with an electronic structure similar to that of Pt, making it an ideal material to replace Pt-based catalysts. Therefore, we introduce Mo2C as the second phase into the MoNi4 system, constructing a Mo2C/MoNi4 heterogeneous structure on a nickel substrate to improve the HER performance by modulating the electronic structure of MoNi4.
At the same time, traditional methods (such as hydrothermal methods, electrochemical deposition, etc.) for preparing nickel-based catalysts have limitations such as low yield and small scale, making them unsuitable for large-scale production and commercial application. Consequently, there is an urgent need to explore a low-cost, simple, and scalable synthesis method. To this end, the Metal Functional Materials Research Team has proposed a method for preparing heterojunction catalysts using laser radiation technology (Figure 1). Under the irradiation of high-energy lasers, the organic solvent in the raw materials rapidly carbonizes to form carbon vapor, which reacts with molybdenum powder to produce Mo2C. Simultaneously, under local high-temperature and high-pressure conditions, molybdenum powder reacts with nickel powder to generate the main phase MoNi4, resulting in the Mo2Cx/MoNi4/NM heterogeneous structure (Figure 2).
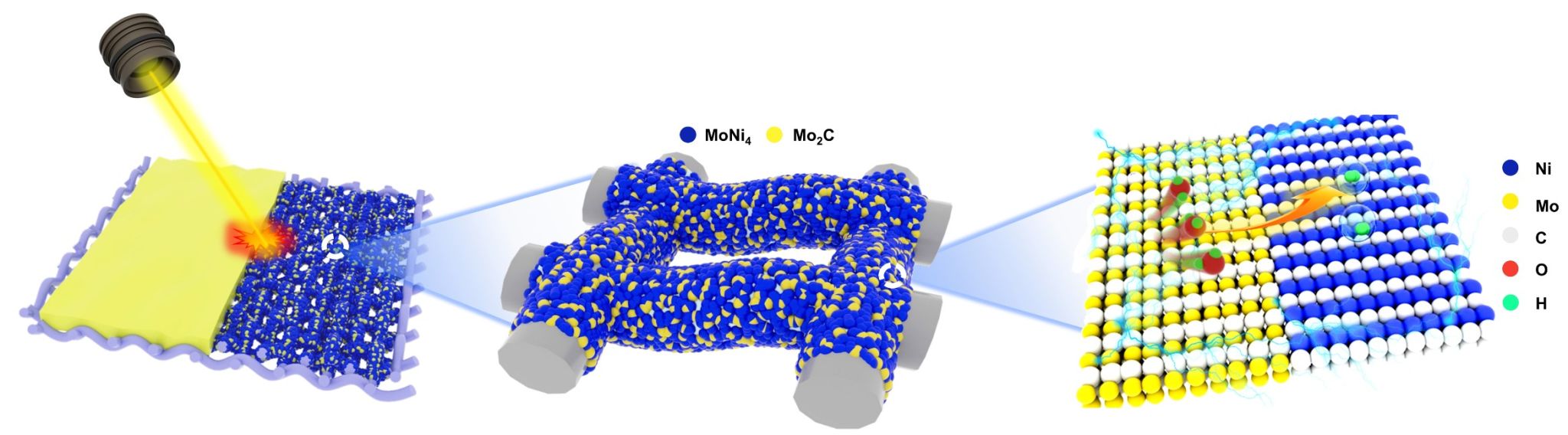
Figure 1. Schematic diagram of the formation of Mo2Cx/MoNi4/NM heterojunction electrodes.
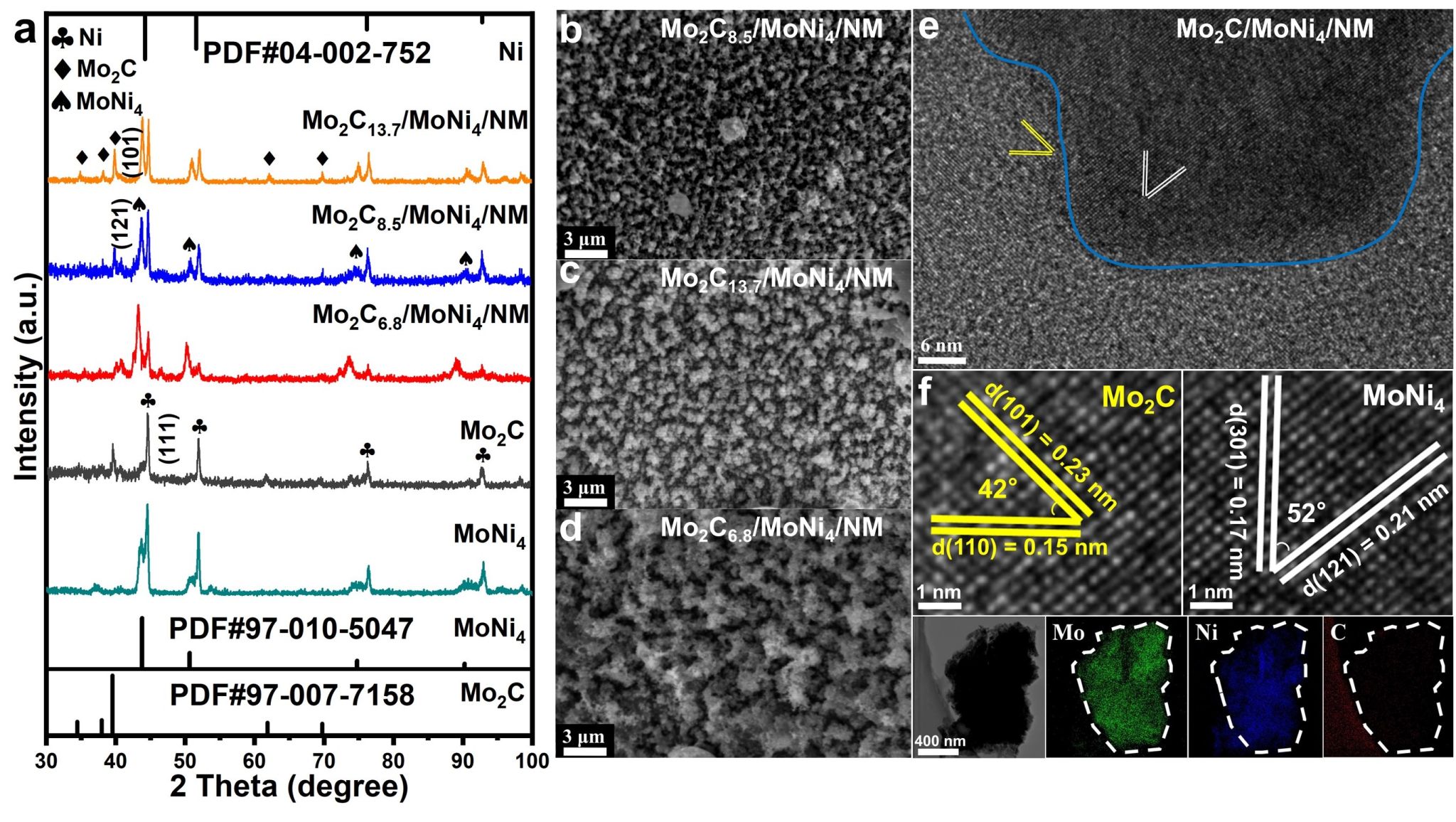
Figure 2. (a) XRD patterns of Mo2C13.7/MoNi4/NM, Mo2C8.5/MoNi4/NM, Mo2C6.8/MoNi4/NM, Mo2C-only, and MoNi4-only after laser treatment. (b) SEM images of Mo2C8.5/MoNi4/NM, (c) Mo2C13.7/MoNi4/NM, and (d) Mo2C6.8/MoNi4/NM. (e) Transmission electron microscopy (TEM) image of Mo2C/MoNi4/NM. (f) HRTEM image of Mo2C/MoNi4/NM and (g) EDS mapping image of Mo2C/MoNi4/NM.
In this study, the laser radiation technology we proposed has advantages such as fast processing speed and low production cost. The high-energy laser can achieve metallurgical bonding at the interface between the catalyst coating and the substrate, ensuring the long-term durability of the electrodes. The experimental results, as shown in Figure 3, indicate that Mo2C8.5/MoNi4/NM possesses an extremely low HER overpotential (η10 = 49.5 mV), comparable to that of the state-of-the-art 20 wt.% Pt/C electrocatalyst. Its Tafel slope is minimal (44.35 mV dec⁻¹), and it has the lowest charge transfer resistance (Rct = 5.969 Ω), alongside the largest specific surface area and electrochemically active surface area, indicating that the catalyst facilitates faster charge transfer rates and more favorable kinetics during the HER process.
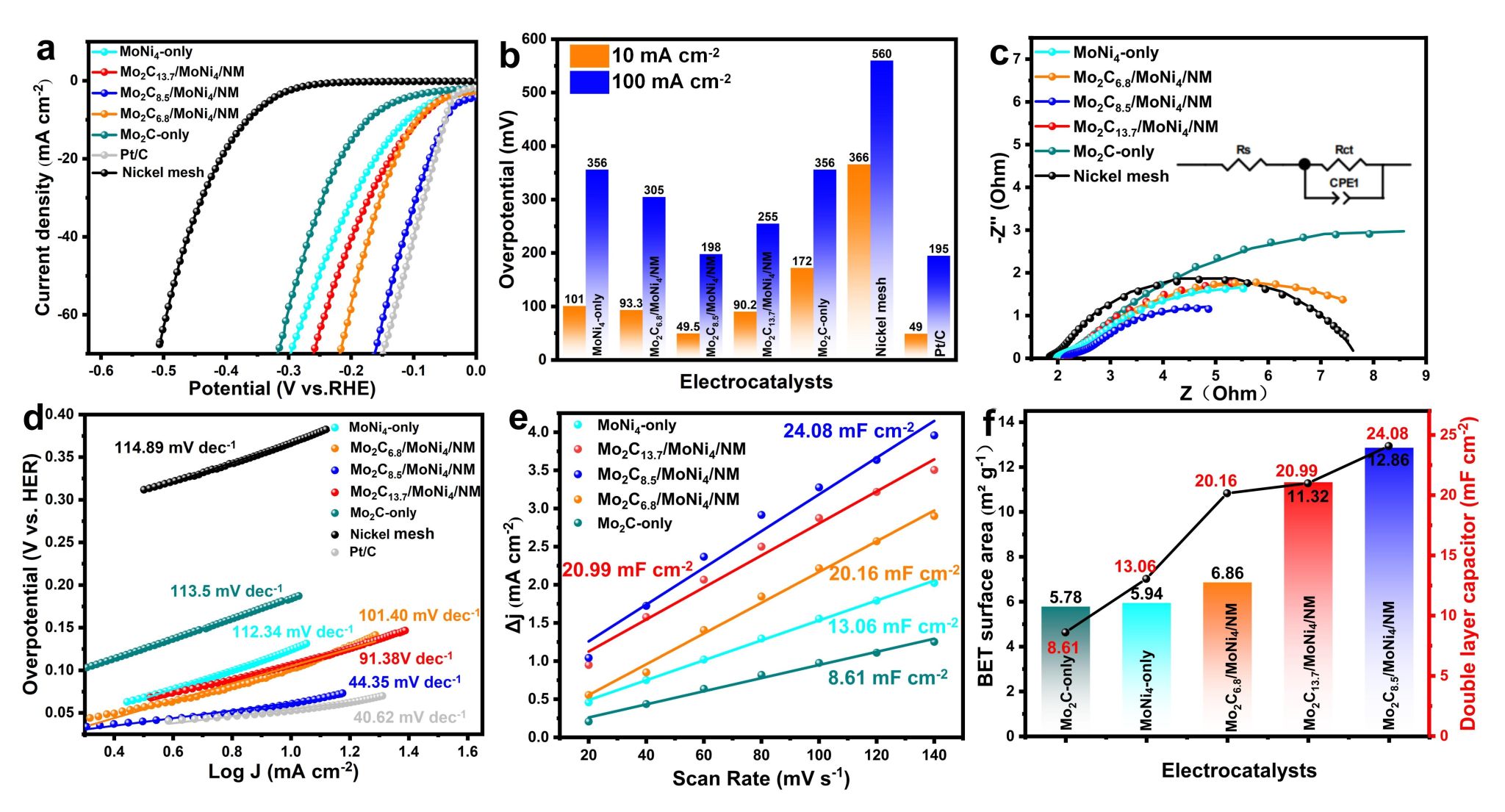
Figure 3. (a) Linear sweep voltammetry (LSV) curves; (b) Comparison of η10 and η100 for different catalysts; (c) Comparison of electrochemical impedance spectroscopy (EIS) for HER; (d) Tafel slopes of MoNi4-only, Mo2C6.8/MoNi4/NM, Mo2C8.5/MoNi4/NM, Mo2C13.7/MoNi4/NM, Mo2C-only, Nickel mesh, and Pt/C; (e) Cdl values inferred from CV curves; (f) BET surface area and Cdl values.
The results of X-ray Photoelectron Spectroscopy (XPS) analysis indicate that the presence of the heterogeneous structure leads to the transfer of electrons from Ni in MoNi4 to Mo in Mo2C. The strong electronic interaction between Mo and Ni causes Ni to carry a positive charge, demonstrating that a small amount of Mo2C doping can effectively modulate the electronic structure of MoNi4. Theoretical calculations show that the introduction of Mo2C lowers the d-band center of the Ni sites, which serve as the main active centers in Mo2C8.5/MoNi4/NM (strong electronic interaction makes Ni positively charged, causing the d-band center (εd) to shift downwards), thereby reducing its excessively strong H* adsorption energy and facilitating the desorption of H2 (Figure 4). Moreover, the hydrogen adsorption free energy (ΔGH* = -0.37 eV) at the heterojunction is significantly lower than that of the two single-phase materials (MoNi4 = -0.51 eV; Mo2C = -1.26 eV), indicating that the construction of the heterojunction can enhance H* adsorption and accelerate H2 desorption, thereby improving HER activity. This confirms the synergistic effect of Mo2C and the MoNi4 alloy in the HER process.
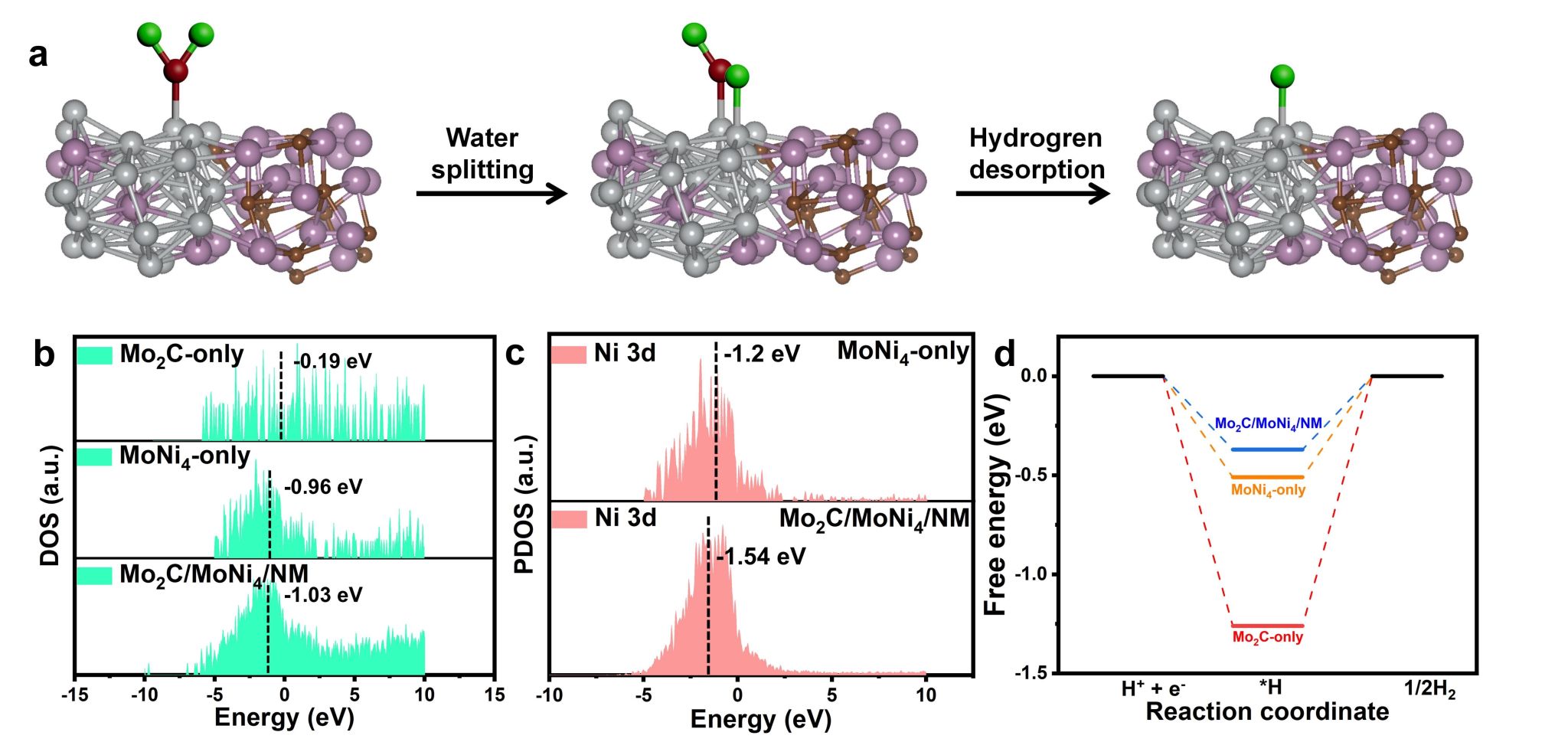
Figure 4. (a) Schematic diagram of the reaction mechanism on Mo2C/MoNi4/NM (gray, blue, brown, red, and green spheres represent Ni, Mo, C, O, and H atoms, respectively). (b) Density of states (DOS) for Mo2C-only, MoNi4-only, and Mo2C/MoNi4/NM. (c) Projected density of states (PDOS) for different catalysts.
Additionally, the catalyst can maintain a stable overpotential for up to 100 hours at a high current density of 100 mA cm⁻². Therefore, the successful preparation of Mo2C/MoNi4 heterojunction electrodes through a simple laser radiation technique represents a feasible approach for large-scale, low-cost production of non-noble metal hydrogen evolution catalysts. This paves the way for the scaled production of electrodes that possess comparable HER activity and stability to Pt/C catalysts. The entire synthesis process provides favorable conditions for multifunctional catalysts in industrial applications. This work will offer valuable insights and guidance on the synergistic effects between two types of water-splitting application architectures.
Paper link: https://advanced.onlinelibrary.wiley.com/doi/10.1002/adfm.202423549
DOI: 10.1002/adfm.202423549